| A B C D E F G H I J K L M N O P Q R S T U V W X Y Z # |
RESPIRATORY VIRUS PANEL BY BIOFIRE FILM ARRAY
Test CodeLAB1897
Includes
Adenovirus, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, SARS-CoV-2, Human Metapneumovirus, Influenza A, Influenza A subtype H1, Influenza A subtype H3, Influenza A subtype H1-2009, Influenza B, Parainfluenza Virus 1, Parainfluenza Virus 2, Parainfluenza Virus 3, Parainfluenza Virus 4, Human Rhinovirus/Enterovirus, Respiratory Syncytial Virus, Bordetella pertussis, Bordetella parapertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae.
Preferred Specimen
Nasopharyngeal swab (flocked) in approximately 3 mL UniversalTransport Media (UTM), or saline.
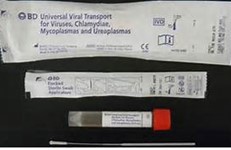

Minimum Volume
Nasopharyngeal swab: 1 swab containing 1.0 mL fluid/media
Transport Temperature
Refrigerated
Specimen Stability
Ambient: 4 hours
Refrigerated: 3 days
Frozen: 30 days
Refrigerated: 3 days
Frozen: 30 days
Reject Criteria (Eg, hemolysis? Lipemia? Thaw/Other?)
Specimens in leaking containers will not be tested. Dry swabs, calcium alginate swabs, samples in bacterial transport systems, and other body fluids will not be tested. Repeat testing within 7 day period is not accepted.
Methodology
Multiplex Polymerase Chain Reaction (PCR) using BioFire film array
Setup Schedule
Sunday-Saturday
Reference Range
Not detected
Performing Laboratory
West Virginia University Hospital, Inc.
Berkeley Medical Center Braxton County Memorial Camden Clark Medical Center Garrett Regional Medical Center Jackson General Hospital Jefferson Medical Center United Hospital Center Uniontown Hospital

