| A B C D E F G H I J K L M N O P Q R S T U V W X Y Z # |
BLOOD PARASITE SCAN (MALARIA STAIN GROUP)
Test CodeLAB1230023
CPT Codes
87207, 87015
Includes
Test Includes: Thick and thin stain of malariaand percent parasitemia for positive results.
Preferred Specimen
One 3 mL lavender top tube
Minimum Volume
2 mL blood
Instructions
Slides must be made on alcohol cleaned slides within 90 minutes of collection. Prepare 6 slides of each thin and thick slide. For thin slides, make bullet shape peripheral prep. For thick slides, place a drop of blood on slide and smear to approximately size of dime, should be able to read through the blood.
STANDARD SMEAR PREPARATION:
NOTE: Slides must be prepared within 90 minutes of collection.
1.Retrieve ethanol cleaned glass slides from storage. If none are available, prepare slides as follows:
STANDARD SMEAR PREPARATION:
NOTE: Slides must be prepared within 90 minutes of collection.
1.Retrieve ethanol cleaned glass slides from storage. If none are available, prepare slides as follows:
- Add ethanol to a Coplin jar, and add glass slides.
- Remove slides individually and wipe gently with a Kimwipe lint free paper.
- Place slides on a slide rack to completely dry.
- After ethanol cleaning, store slides in a dust free container.
- Make and label 6 routine blood smears on ethanol cleaned slides.
- Label slides with accession number, patient name with first initial, date and THIN
- Allow smears to completely.
- Fix the remaining slides in absolute methanol for 2 minutes
-
- Make and label 6 thick blood smears on ethanol-cleaned slides.
- Label slides with accession number, patient name with first initial, date and THICK.
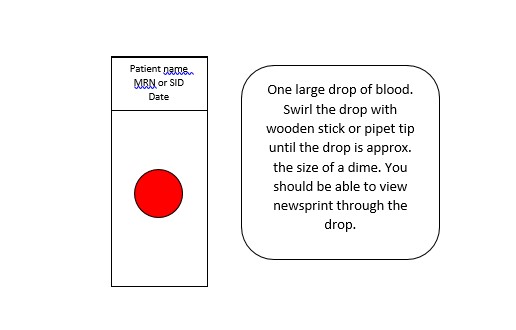
- Using two wooden applicator sticks, transfer a medium sized drop of blood to a slide.
- Use the sticks to carefully spread the drop of blood into a dimesized circle (10-12 mm).
- Remove any bubbles that may form, and remove any fibrous material left from the applicator sticks.
- Use caution to not make the smear too thick or it will detach from the slide. A well made smear will allow newsprint to be read
through it. - Transfer the thick smears to a slide folder, with the smear side up and uncovered. Allow slides to air dry 6-8 hours. The thick
smears must be absolutely dry before lysis, or else the blood smear is likely to detach from the slide. Do not fix the thick
smears.
- Once slides are dry, they can be sent via courier. They do NOT have to sit and completely dry for the 6-8 hours before transport
Transport Temperature
Ambient (lavender top tube and slides)
Specimen Stability
Unstable, send immediately tolaboratory. Slides must be made within 90 minutes of collection time.
Reject Criteria (Eg, hemolysis? Lipemia? Thaw/Other?)
Clotted specimen or specimen greater than 90 minutes old. too few or not both thick and thin smears.
Any slides submitted for testing or review must be labeled with two patient identifiers. Acceptable identifiers include Name, MRN, Birth date, Accession/SID.
Any slides submitted for testing or review must be labeled with two patient identifiers. Acceptable identifiers include Name, MRN, Birth date, Accession/SID.
Methodology
Manual Giemsa Stain
Setup Schedule
Sunday-Saturday
Reference Range
Negative
Performing Laboratory
West Virginia University Hospital, Inc.
Camden Clark Medical Center

