|
|
| A B C D E F G H I J K L M N O P Q R S T U V W X Y Z # |
BK VIRUS, PCR, URINE QUANTITATIVE
Test CodeLAB304251
Includes
Viral load value and Log 10 value
Preferred Specimen
Urine
Minimum Volume
1.0 mL
Other Acceptable Specimens
Non applicatble
Instructions
Urine must be received in the Molecular Diagnostics Laboratory within 24 hours. If the urine is unable to make it to the Molecular Diagnostics Laboratory, the urine must be stablized with the coabs PCR Media tube (WOrkday order # 330644). Urine is added to stablizing liquid until it is within the designated marking on the tube.
How to add urine to the cobas PCR media tube:
1. Use pipette to pull up urine from the sterile urine cup.
2. Transfer urine in the cobas PCR media and fill to within the designated fill window.
3. Place the cap onto the cobas PCR media tube securely.
4. Invert securely capped cobas PCR media tube to mix stabilizing fluid and urine.
5. Store/transport urine filled cobas PCR media tube at 2-8 degrees Celsius.
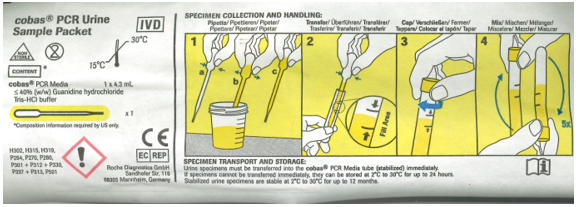
How to add urine to the cobas PCR media tube:
1. Use pipette to pull up urine from the sterile urine cup.
2. Transfer urine in the cobas PCR media and fill to within the designated fill window.
3. Place the cap onto the cobas PCR media tube securely.
4. Invert securely capped cobas PCR media tube to mix stabilizing fluid and urine.
5. Store/transport urine filled cobas PCR media tube at 2-8 degrees Celsius.

Transport Temperature
Refrigerated: 2-8°C
Specimen Stability
Urine in sterile cupL 2-8°C for 24 hours
Urine stabilized in the cobas PCR Media tube; 2-8°C for 90 days
Urine stabilized in the cobas PCR Media tube; 2-8°C for 90 days
Reject Criteria (Eg, hemolysis? Lipemia? Thaw/Other?)
Urine that exceeds 24 hours and is not in the cobas PCR Media Tube.
Non-Validated specimen type
broken specimen container
specimen container not properly labelled
Non-Validated specimen type
broken specimen container
specimen container not properly labelled
Methodology
Polymerase Chaing Rection (PCR)
Setup Schedule
Monday-Wednesday-FRiday
Clinical Significance
To monitor BKV infections in kidney transplant patients and hematopoietic stem cell transplant patients.
Performing Laboratory
West Virginia University Hospital, Inc.

