|
|
| A B C D E F G H I J K L M N O P Q R S T U V W X Y Z # |
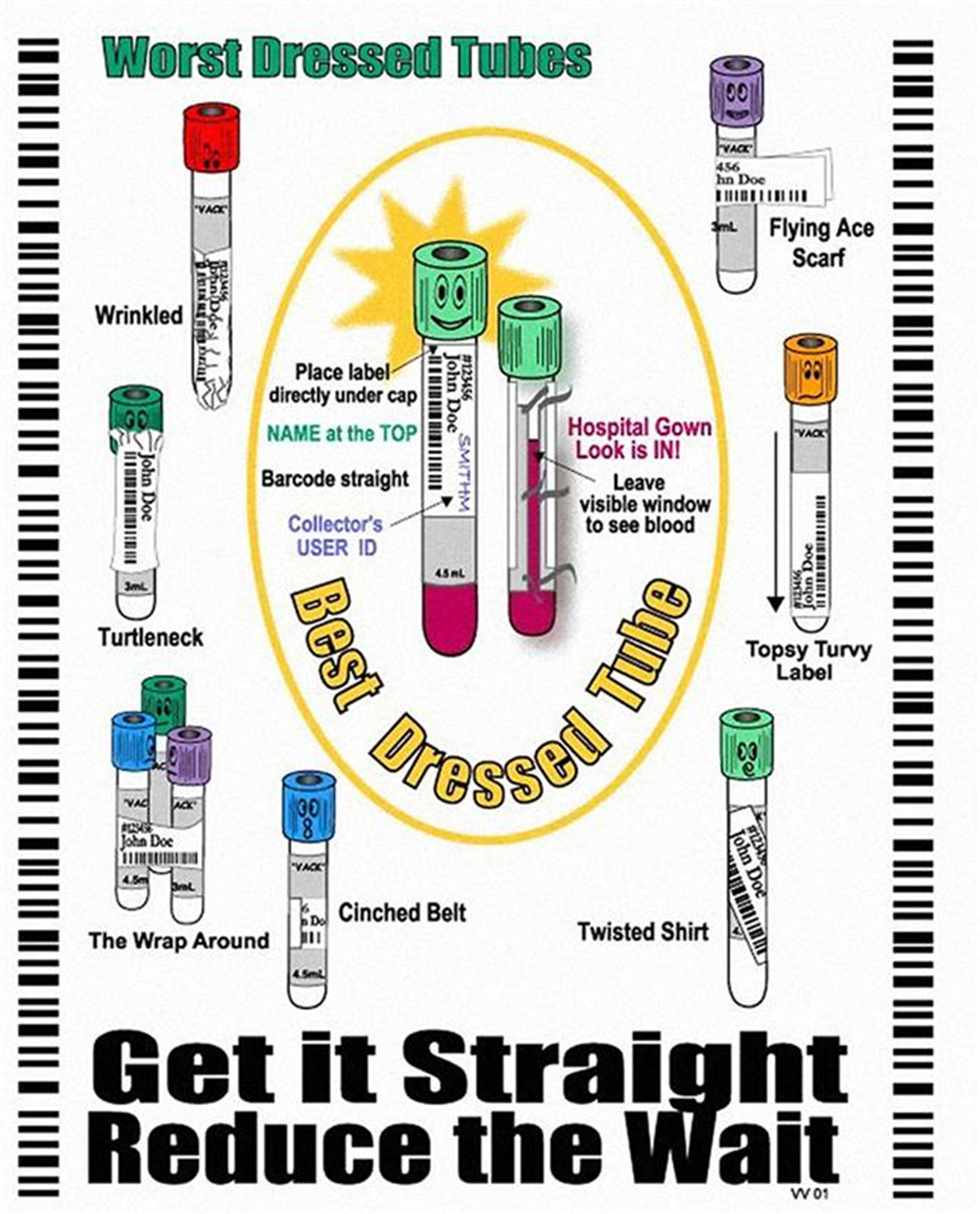
1.04- Collection Tube Chart & Specimen Label Placement
Messagelist of collection tubes
Order of tube draw for Blood collections:
Yellow top SPS tubes for Blood Cultures
Aerobic Plus Bactec FX bottle (blue for aerobic) for Blood Culture
Anaerobic Lytic Bactec FX bottle (magenta for anaerobic) for Blood Culture
Blue top tube for Coagulation testing*
Gold SST or Red/Grey SST (Marble or Tiger top tube)
Plastic Plain Red (no gel)
Heparin Tube (eg, Green top tube, Light Green top tube)
EDTA Tube (eg, Lavender, Pink top tube)
Grey top tube
Yellow (ACD) top tube
All other tubes containing an anti-coagulant additive.
* When using a winged blood collection set for venipunture and the coagulation tube is the only tube or the first tube to be drawn, a discard tube should be drawn first. The discard tube must be used to fill the blood collection set tubing's "dead space" with blood but the discard tube does not need to be completely filled. This important step will ensure the proper blood-to-additive ratio of the blood specimen. The discard tube should be a non-additive or coagulation tube.
References: BD Diagnostics Order of Draw for Multiple Tube Collections 01/2010
MINIMIZING SPECIMEN VOLUME
The Laboratory makes every effort to minimize the amount of blood required by use of state-of-the-art instrumentation and methods. Please do not hesitate to contact the appropriate testing section if there are problems with seriously ill or hard-to-draw patients. The laboratory will make every effort to add additional tests to an existing specimen in order to minimize the specimen volume collected from the patient.
A full collection tube should be submitted in all cases. For blood collection tubes containing anticoagulants, a full tube must be submitted. Unless further information is provided in VISTA Test Description, you may expect to get up to 15 routine tests from a full gold top tube. If ordering multiple Hepatitis tests, then 2 gold top tubes will be needed. If drawing tests for multiple laboratory areas, contact each laboratory department to determine if pouring off and sharing a specimen is a viable option.
INDWELLING LINES/CATHETERS
Because it may be normal practice to flush lines with a solution of heparin or saline to reduce the risk of thrombosis, lines must be cleared of this fluid before blood specimens can be drawn for diagnostic testing.We recommend that the first 5mL of blood or six times the volume of the line (whichever volume is greater) be discarded before a specimen is obtained for analysis to ensure the actual specimen is not diluted with the flush solution. The discard tube should be a non-additive or coagulation tube.
NOTE: Laboratory personnel will not draw blood from indwelling lines, catheters or cardiovascular (arterial, central venous) lines.
FISTULA/ BLOOD SPECIMENS FROM THE CANNULA
Routinely, the fistula arms should not be used for blood drawing.A cannula is used as an access for dialyzing and for blood drawing in kidney patients. For convenience in obtaining blood specimens from patients who have a cannula, a "T" connector is used.Drawing blood from the cannula with a "T" tube on kidney transplant or dialysis patients should be done only by the transplant or dialysis teams.Timing of when the specimen is obtained may impact the specimen integrity. If specimen is obtained at the initiation of use of this line it is necessary to draw a discard tube to avoid heparin dilution effect. We recommend that the first 5mL of blood or six times the volume of the line (whichever volume is greater) be discarded before a specimen is obtained for analysis to ensure the actual specimen is not diluted with the flush solution. The discard tube should be a non-additive or coagulation tube.
INTRAVENOUS FLUIDS
When a patient is being administered an IV infusion do not draw blood from that arm.Look for a blood‑drawing site in the opposite arm.Occasionally, an IV will be running in both arms or the other arm may not be accessible and no other site can be found.Satisfactory samples may be drawn below the IV by following these procedures:
1.Turn off the IV for at least 2 minutes before venipuncture.
2.Apply tourniquet below the IV site.
3.Select a vein other than the one with the IV.
4.Perform venipuncture.Draw 5 ml of blood and discard before drawing test specimens. The discard tube should be a non-additive or coagulation tube
LEAK PROOF SCREW CAPPED CONTAINER:
All Specimens for Microbiology, Cytology and Histology
NOTE: Large specimens require special handling.
ANAEROBIC TRANSPORT CONTAINERS
All Specimens for Anaerobic Culture.
NOTE: ALL SPECIMENS ARE TO BE PLACED IN A LEAK PROOF CONTAINER AND BAGGED (PLASTIC BAGGIE) PRIOR TO TRANSPORT.
THERAPEUTIC DRUG MONITORING
Recommended times for specimen collection for commonly ordered in-house therapeutic drugs are reflected in the following table:

| Color | Characteristics | Volume of Draw | Additive | Minimum Volume |
| Pink | Plasma for Blood Bank | 6 mL | K2EDTA | 6 mL |
| Red | Clot activator, serum for therapeutic rugs & toxicology (Not for Blood Bank use)Do Not Mix or Invert | 4 mL | None | N/A |
| Gold | Polymer gel & clot activator, serum. Used for general chemistry, endocrinology, immunology & sendout tests. Tube should be gently inverted 3-4 times after being filled | 5 mL | None | N/A |
| Lavender | Contains K2EDTA for whole blood collection. Used for hematology tests (CBC, ESR, etc.) also used for specialized chemistry testing (BNP, Ammonia). Tube should be gently inverted 8-10 times after being filled. | 4 mL | K2EDTA | 2 mL |
| Green (dark) | Contains sodium heparin for collection of whole blood & bone marrow.Used for ionized calcium and PTH. Tube should be gently inverted 8-10 times after being filled. | 2 mL or 7 mL | 100 USP Units of Sodium Heparin | N/A |
| Green (light) | Contains lithium heparin as the anticoagulant. Used for general chemistry & troponin testing.Tube should be gently inverted 8-10 times after being filled. | 4.5 mL | Lithium Heparin | N/A |
| Blue (light) | Contains buffered sodium citrate for coagulation studies.Tube should be gently inverted 8-10 times after being filled. | 2.7 mL | 0.109 M Sodium Citrate | 2.43 mL |
| Grey | Contains anticoagulant and glycolytic inhibitor, for Lactic Acid testing. Also used for Cocaine testing Tube should be gently inverted 8-10 times after being filled. | 7 mL | 15 mg Potassium Oxalate & 12.5 mg Sodium fluoride | N/A |
| Yellow | Contains acid citrate dextrose (ACD) solution for the preservation of red blood cells. Used for flow ctyometry analysis. Tube should be gently inverted 8-10 times after being filled. | 8.5 mL | 1.5 MI ACD Solution | N/A |
| Yellow (SPS) | Contains sodium polyanetholesulfonate & sodium chloride, used for fungae or mycobacterial blood/fluid specimens.Tube should be gently inverted 8-10 times after being filled. | 8 mL | Sodium Polyanetholesulfonate and Sodium Chloride | N/A |
| Royal Blue | No additive, used for trace element analysis of serum | 7 mL | None (silicon coated) | N/A |
| Royal Blue | Contains K2EDTA used for trace elements analysis of plasma and or whole blood.Tube should be gently inverted 8-10 times after being filled. | 7 mL | K2EDTA | N/A |
Order of tube draw for Blood collections:
Yellow top SPS tubes for Blood Cultures
Aerobic Plus Bactec FX bottle (blue for aerobic) for Blood Culture
Anaerobic Lytic Bactec FX bottle (magenta for anaerobic) for Blood Culture
Blue top tube for Coagulation testing*
Gold SST or Red/Grey SST (Marble or Tiger top tube)
Plastic Plain Red (no gel)
Heparin Tube (eg, Green top tube, Light Green top tube)
EDTA Tube (eg, Lavender, Pink top tube)
Grey top tube
Yellow (ACD) top tube
All other tubes containing an anti-coagulant additive.
* When using a winged blood collection set for venipunture and the coagulation tube is the only tube or the first tube to be drawn, a discard tube should be drawn first. The discard tube must be used to fill the blood collection set tubing's "dead space" with blood but the discard tube does not need to be completely filled. This important step will ensure the proper blood-to-additive ratio of the blood specimen. The discard tube should be a non-additive or coagulation tube.
References: BD Diagnostics Order of Draw for Multiple Tube Collections 01/2010
MINIMIZING SPECIMEN VOLUME
The Laboratory makes every effort to minimize the amount of blood required by use of state-of-the-art instrumentation and methods. Please do not hesitate to contact the appropriate testing section if there are problems with seriously ill or hard-to-draw patients. The laboratory will make every effort to add additional tests to an existing specimen in order to minimize the specimen volume collected from the patient.
A full collection tube should be submitted in all cases. For blood collection tubes containing anticoagulants, a full tube must be submitted. Unless further information is provided in VISTA Test Description, you may expect to get up to 15 routine tests from a full gold top tube. If ordering multiple Hepatitis tests, then 2 gold top tubes will be needed. If drawing tests for multiple laboratory areas, contact each laboratory department to determine if pouring off and sharing a specimen is a viable option.
INDWELLING LINES/CATHETERS
Because it may be normal practice to flush lines with a solution of heparin or saline to reduce the risk of thrombosis, lines must be cleared of this fluid before blood specimens can be drawn for diagnostic testing.We recommend that the first 5mL of blood or six times the volume of the line (whichever volume is greater) be discarded before a specimen is obtained for analysis to ensure the actual specimen is not diluted with the flush solution. The discard tube should be a non-additive or coagulation tube.
NOTE: Laboratory personnel will not draw blood from indwelling lines, catheters or cardiovascular (arterial, central venous) lines.
FISTULA/ BLOOD SPECIMENS FROM THE CANNULA
Routinely, the fistula arms should not be used for blood drawing.A cannula is used as an access for dialyzing and for blood drawing in kidney patients. For convenience in obtaining blood specimens from patients who have a cannula, a "T" connector is used.Drawing blood from the cannula with a "T" tube on kidney transplant or dialysis patients should be done only by the transplant or dialysis teams.Timing of when the specimen is obtained may impact the specimen integrity. If specimen is obtained at the initiation of use of this line it is necessary to draw a discard tube to avoid heparin dilution effect. We recommend that the first 5mL of blood or six times the volume of the line (whichever volume is greater) be discarded before a specimen is obtained for analysis to ensure the actual specimen is not diluted with the flush solution. The discard tube should be a non-additive or coagulation tube.
INTRAVENOUS FLUIDS
When a patient is being administered an IV infusion do not draw blood from that arm.Look for a blood‑drawing site in the opposite arm.Occasionally, an IV will be running in both arms or the other arm may not be accessible and no other site can be found.Satisfactory samples may be drawn below the IV by following these procedures:
1.Turn off the IV for at least 2 minutes before venipuncture.
2.Apply tourniquet below the IV site.
3.Select a vein other than the one with the IV.
4.Perform venipuncture.Draw 5 ml of blood and discard before drawing test specimens. The discard tube should be a non-additive or coagulation tube
LEAK PROOF SCREW CAPPED CONTAINER:
All Specimens for Microbiology, Cytology and Histology
NOTE: Large specimens require special handling.
ANAEROBIC TRANSPORT CONTAINERS
All Specimens for Anaerobic Culture.
NOTE: ALL SPECIMENS ARE TO BE PLACED IN A LEAK PROOF CONTAINER AND BAGGED (PLASTIC BAGGIE) PRIOR TO TRANSPORT.
THERAPEUTIC DRUG MONITORING
Recommended times for specimen collection for commonly ordered in-house therapeutic drugs are reflected in the following table:
Test Code
Collection Tubes & Label Placement
Alias/See Also
 DO NOT cover the bottom of the bottle. This will cause false positive and/or false negative results.
DO NOT cover the bottom of the bottle. This will cause false positive and/or false negative results. Do not use indented or bent bottles:
Do not use indented or bent bottles:  This is how to correctly place a label on a blood culture bottle:
This is how to correctly place a label on a blood culture bottle:  This is also how to correctly place a label on a blood culture bottle. DO NOT cover the bottles barcode.
This is also how to correctly place a label on a blood culture bottle. DO NOT cover the bottles barcode.  This is all the information needed: Green: Initials of staff drawing bottle Blue: Body site where blood culture was taken from Orange: Time bottle was collected (may also record date)
This is all the information needed: Green: Initials of staff drawing bottle Blue: Body site where blood culture was taken from Orange: Time bottle was collected (may also record date) 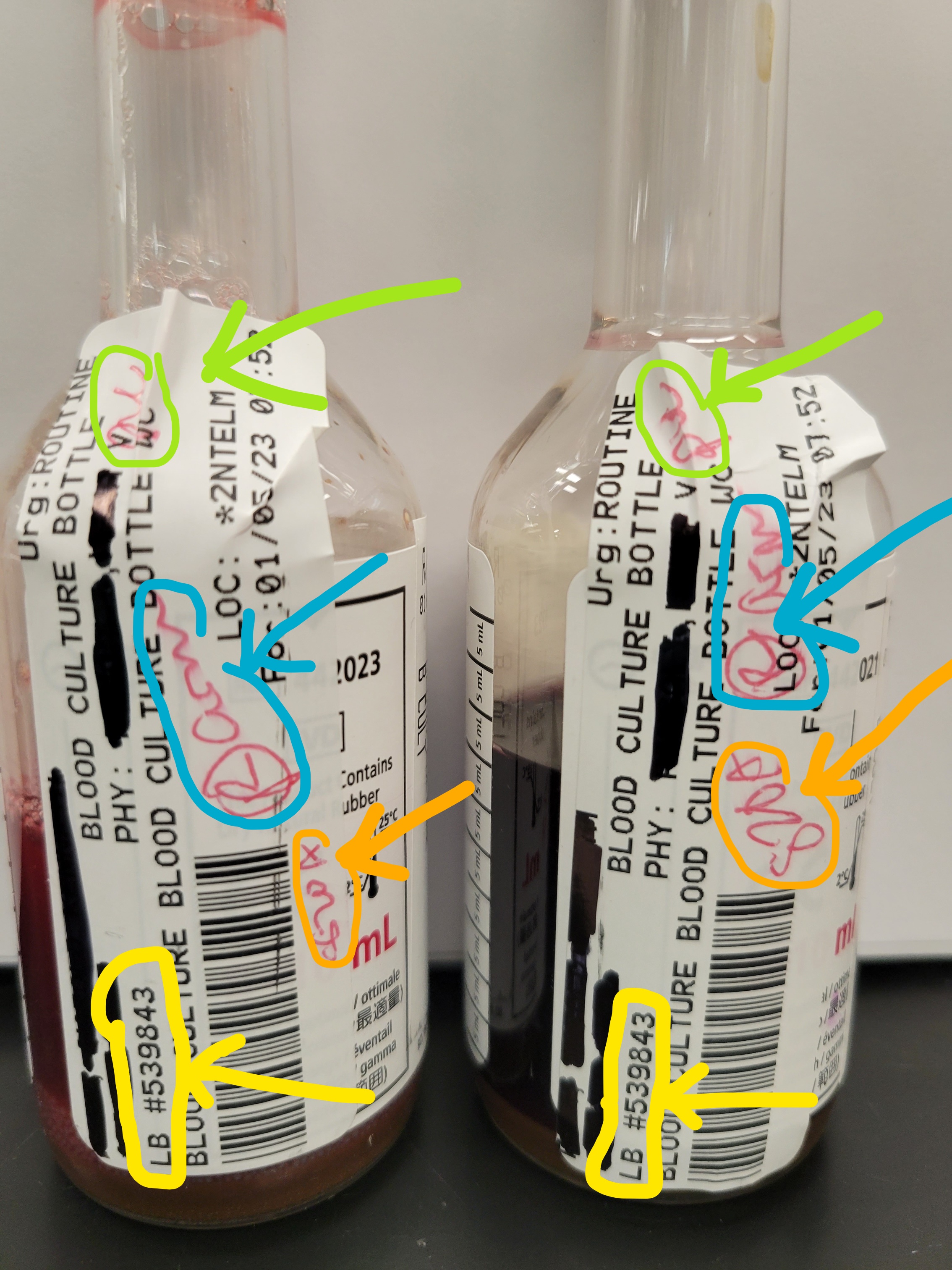
Last Updated: December 23, 2025

